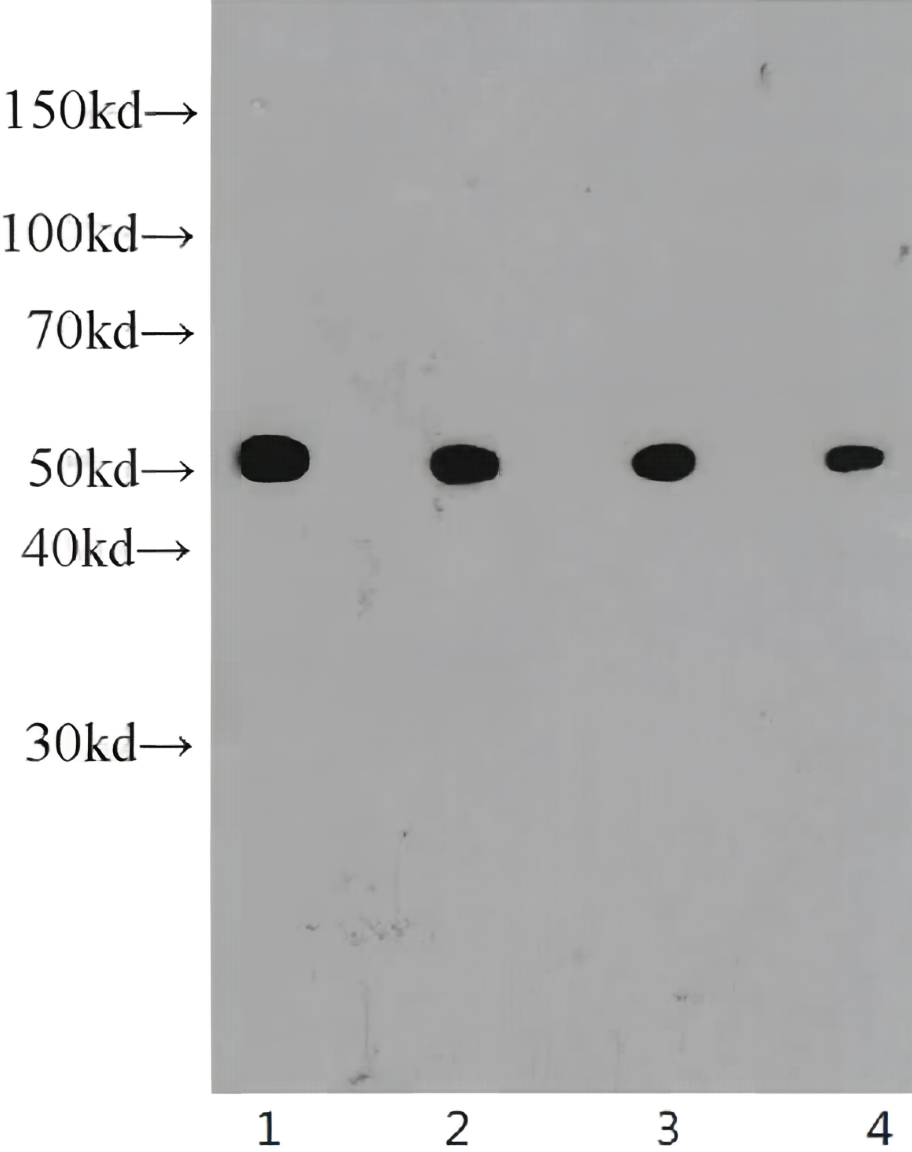
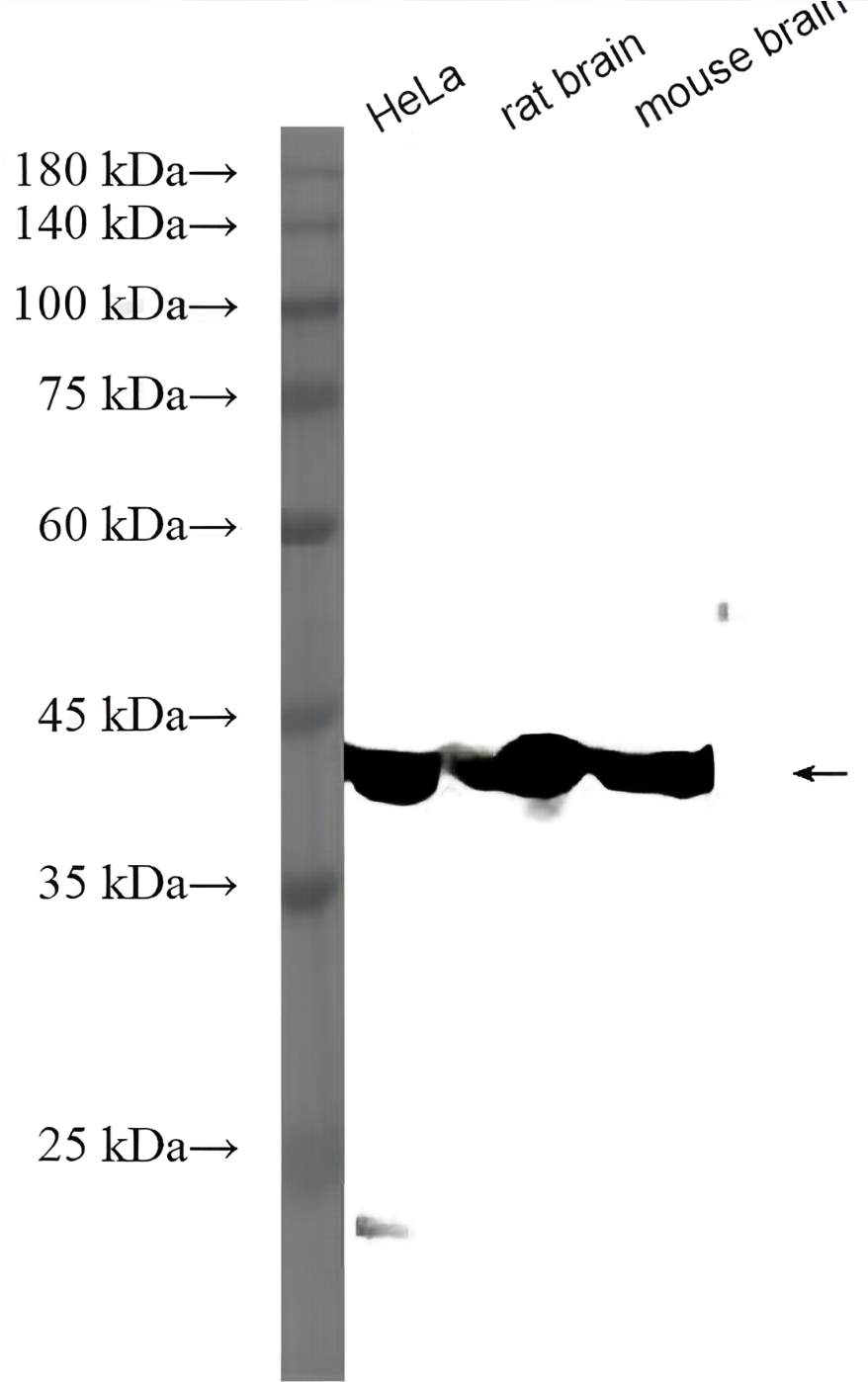
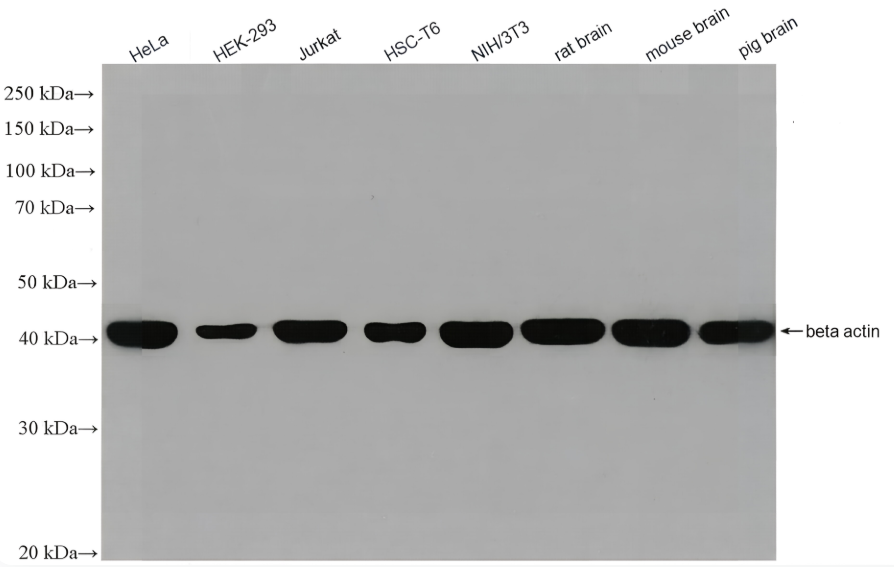
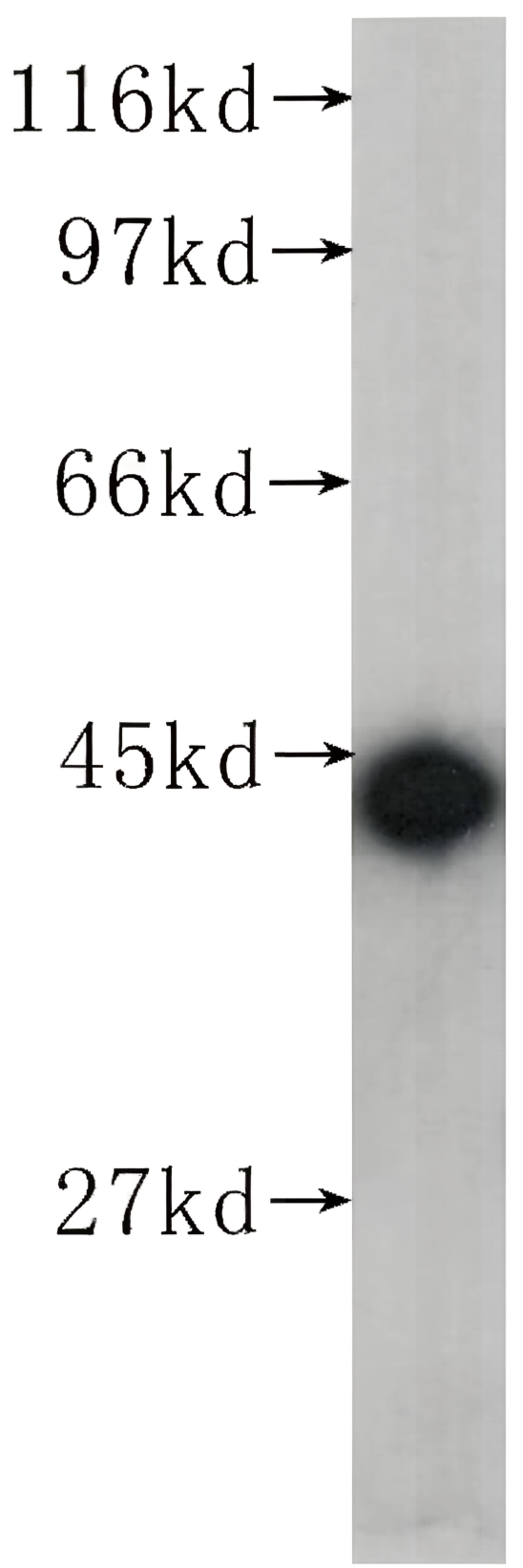
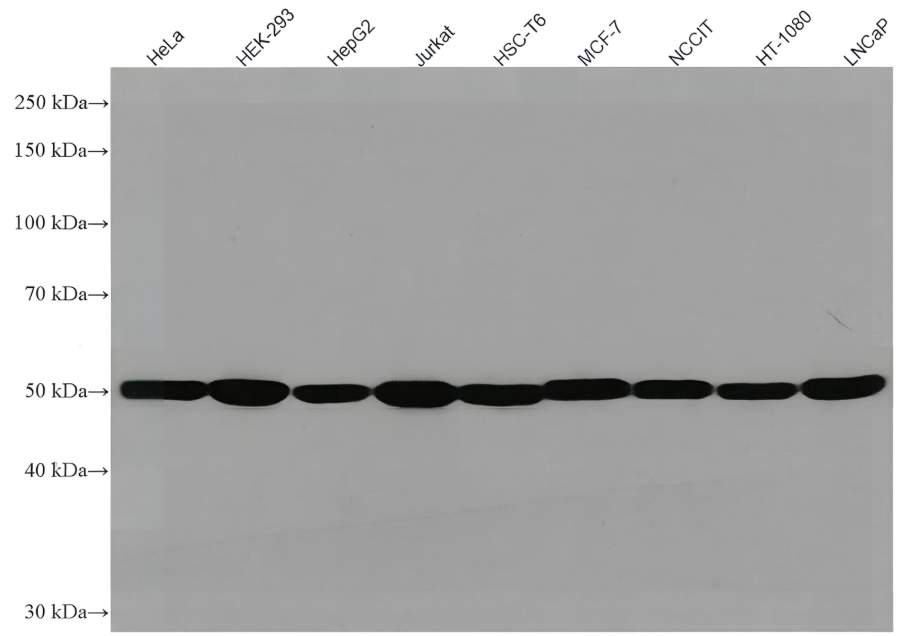
Recombinant Tetanus Toxin C Fragment
CAT.NO. : ARP6777
RMB Please choose
RMB Please choose
Size:
Trail, Bulk size or Custom requests Please contact us
*产品价格可能会有所调整,请以品牌方官网实时更新的价格为准,以确保准确性。
Background
Tetanus toxin is a potent neurotoxin secreted by the anaerobic bacterium Clostridium tetani and is the compound responsible for the symptoms of tetanus. The toxin consists of two polypeptide chains connected by a disulfide linkage. The heavy chain contains two regions of interest: a binding domain and a translocation domain. The binding domain allows the toxin to be taken up by neurons upon binding to gangliosides on nerve endings. Upon reaching inhibitory neurons via axonal and transsynaptic transport, the translocation domain allows the toxin to enter the cytosol. The light chain is a zinc endopeptidase, activated by enzymes in the cytosol that cleaves the disulfide bond between the heavy and light chain. Once activated, the light chain cleaves synaptobrevin 2, blocking release of inhibitory transmitters GABA and glycine to motor neurons, causing the prolonged muscle transactions symptomatic of tetanus. Enzymatic cleavage of tetanus toxin by papain generates two main molecules: 1) the light chain linked to the N-terminal portion of the heavy chain containing the translocation domain, and 2) the C-fragment, or the C-terminal portion of the heavy chain, which contains the binding domain. C-fragment is considered nontoxic and, when recombinantly produced, contains no trace of intact toxin.
In vitro, tetanus toxin C-fragment (TTC) is an efficient mucosal adjuvant and carrier molecule for the generation of mucosal antibody responses and/or induction of systemic T-cell tolerance to linked antigens. TTC is also used for tract tracing in neurological research, taking advantage of its high affinity to GT1B gangliosides and retrograde transport. Recombinant tetanus toxin C-fragment is a 51.6kDa globular protein containing 451 amino acids.
 New Products
New Products