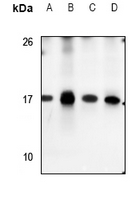
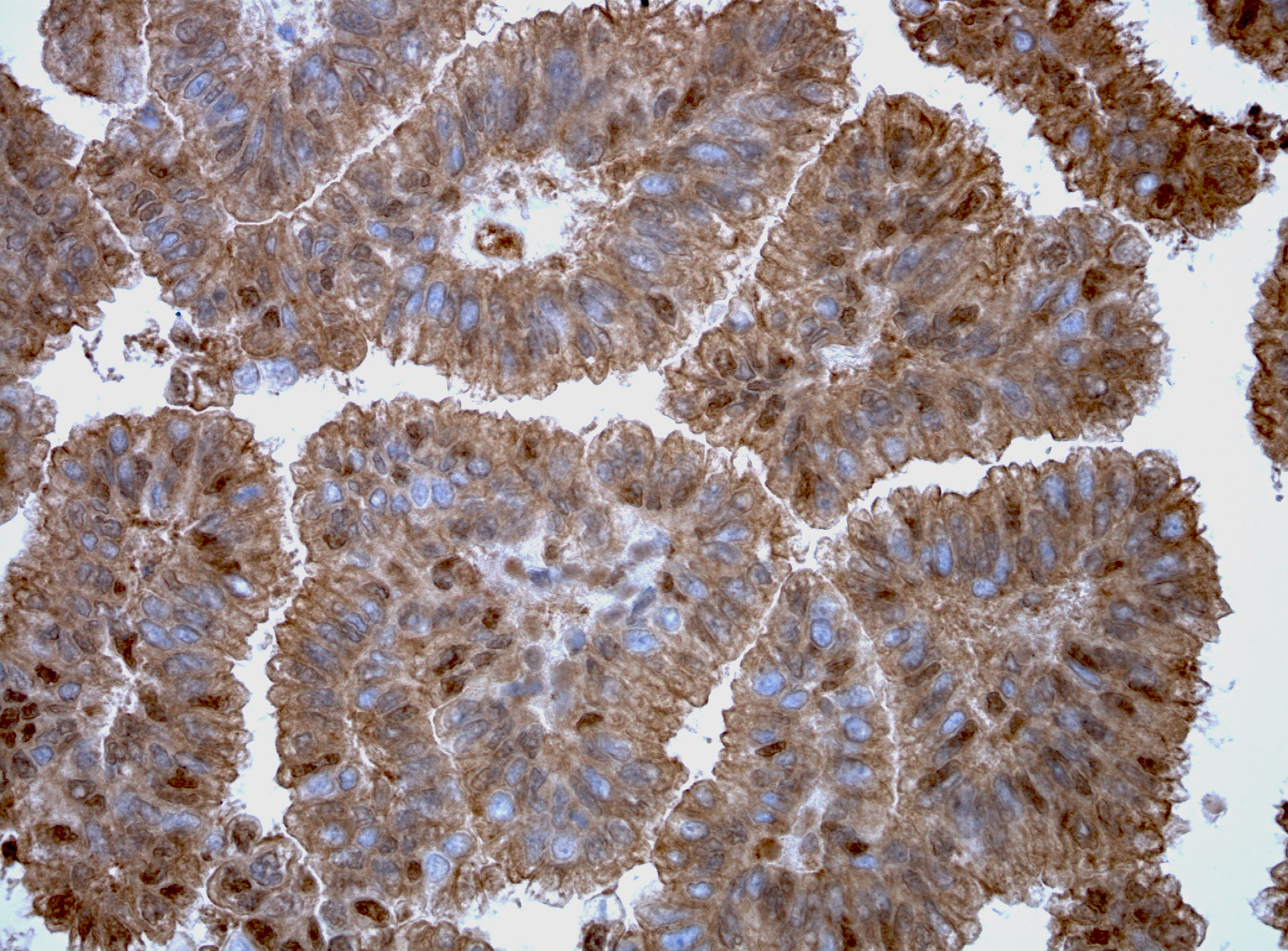
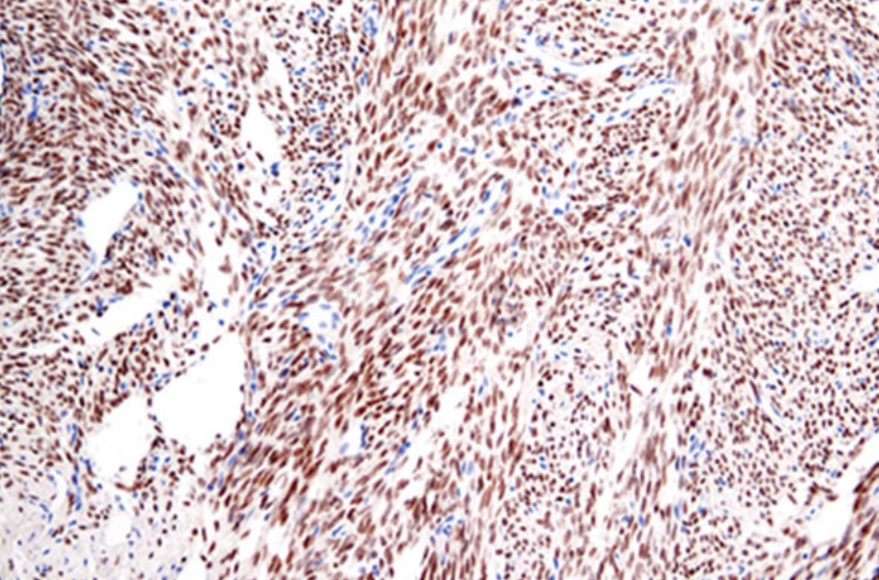
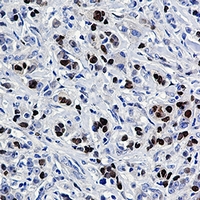
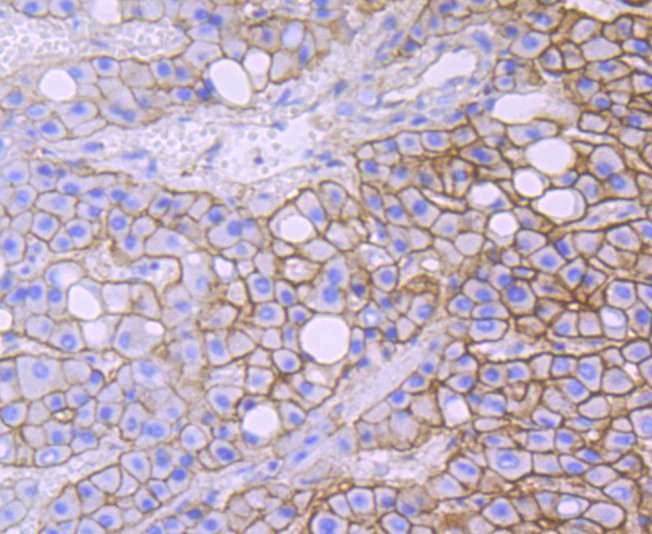
anti-HSF1 antibody [4B4]
CAT.NO. : ARG62504
US$ Please choose
US$ Please choose
Size:
Trail, Bulk size or Custom requests Please contact us
*产品价格可能会有所调整,请以品牌方官网实时更新的价格为准,以确保准确性。
概述
| 产品描述 | Rat Monoclonal antibody [4B4] recognizes HSF1 |
|---|---|
| 反应物种 | Hu, Ms, Rat |
| 应用 | ICC/IF, IP, WB |
| 宿主 | Rat |
| 克隆 | Monoclonal |
| 克隆号 | 4B4 |
| 同位型 | IgG1 |
| 靶点名称 | HSF1 |
| 抗原物种 | Mouse |
| 抗原 | Recombinant mouse HSF1 protein (aa1-503). |
| 抗原表位 | aa 425-439 of mouse HSF1 |
| 偶联标记 | Un-conjugated |
| 別名 | Heat shock transcription factor 1; Heat shock factor protein 1; HSF 1; HSTF 1; HSTF1 |
应用说明
| 应用建议 |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 应用说明 | * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. | ||||||||
| 阳性对照 | LS174T or MAD109 cells |
属性
| 形式 | Liquid |
|---|---|
| 纯化 | Purified Antibody |
| 缓冲液 | 1X PBS and 0.1% Sodium azide |
| 抗菌剂 | 0.1% Sodium azide |
| 浓度 | 0.2 mg/ml |
| 存放说明 | For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. |
| 注意事项 | For laboratory research only, not for drug, diagnostic or other use. |
生物信息
| 数据库连接 | |
|---|---|
| 基因名称 | Hsf1 |
| 全名 | heat shock factor 1 |
| 背景介绍 | The product of this gene is a heat-shock transcription factor. Transcription of heat-shock genes is rapidly induced after temperature stress. Hsp90, by itself and/or associated with multichaperone complexes, is a major repressor of this gene. [provided by RefSeq, Jul 2008] |
| 生物功能 | DNA-binding protein that specifically binds heat shock promoter elements (HSE) and activates transcription. In higher eukaryotes, HSF is unable to bind to the HSE unless the cells are heat shocked. [UniProt] |
| 研究领域 | Cell Biology and Cellular Response antibody; Controls and Markers antibody; Gene Regulation antibody |
| 预测分子量 | 57 kDa |
| 翻译后修饰 | Phosphorylated (PubMed:9499401, PubMed:10359787, PubMed:11583998, PubMed:26159920). Phosphorylated in unstressed cells; this phosphorylation is constitutive and implicated in the repression of HSF1 transcriptional activity (PubMed:8946918, PubMed:8940068, PubMed:9121459, PubMed:16278218). Phosphorylated on Ser-121 by MAPKAPK2; this phosphorylation promotes interaction with HSP90 proteins and inhibits HSF1 homotrimerization, DNA-binding and transactivation activities (PubMed:16278218). Phosphorylation on Ser-303 by GSK3B/GSK3-beta and on Ser-307 by MAPK3 within the regulatory domain is involved in the repression of HSF1 transcriptional activity and occurs in a RAF1-dependent manner (PubMed:8946918, PubMed:8940068, PubMed:9121459, PubMed:9535852, PubMed:10747973, PubMed:12646186). Phosphorylation on Ser-303 and Ser-307 increases HSF1 nuclear export in a YWHAE- and XPO1/CRM1-dependent manner (PubMed:12917326). Phosphorylation on Ser-307 is a prerequisite for phosphorylation on Ser-303 (PubMed:8940068). According to PubMed:9535852, Ser-303 is not phosphorylated in unstressed cells. Phosphorylated on Ser-419 by PLK1; phosphorylation promotes nuclear translocation upon heat shock (PubMed:15661742). Hyperphosphorylated upon heat shock and during the attenuation and recovery phase period of the heat shock response (PubMed:11447121, PubMed:12659875, PubMed:24581496). Phosphorylated on Thr-142; this phosphorylation increases HSF1 transactivation activity upon heat shock (PubMed:12659875). Phosphorylation on Ser-230 by CAMK2A; this phosphorylation enhances HSF1 transactivation activity upon heat shock (PubMed:11447121). Phosphorylation on Ser-326 by MAPK12; this phosphorylation enhances HSF1 nuclear translocation, homotrimerization and transactivation activities upon heat shock (PubMed:15760475, PubMed:27354066). Phosphorylated on Ser-320 by PRKACA/PKA; this phosphorylation promotes nuclear localization and transcriptional activity upon heat shock (PubMed:21085490). Phosphorylated on Ser-363 by MAPK8; this phosphorylation occurs upon heat shock, induces HSF1 translocation into nuclear stress bodies and negatively regulates transactivation activity (PubMed:10747973). Neither basal nor stress-inducible phosphorylation on Ser-230, Ser-292, Ser-303, Ser-307, Ser-314, Ser-319, Ser-320, Thr-323, Ser-326, Ser-338, Ser-344, Ser-363, Thr-367, Ser-368 and Thr-369 within the regulatory domain is involved in the regulation of HSF1 subcellular localization or DNA-binding activity; however, it negatively regulates HSF1 transactivation activity (PubMed:25963659). Phosphorylated on Ser-216 by PLK1 in the early mitotic period; this phosphorylation regulates HSF1 localization to the spindle pole, the recruitment of the SCF(BTRC) ubiquitin ligase complex inducing HSF1 degradation, and hence mitotic progression (PubMed:18794143). Dephosphorylated on Ser-121, Ser-307, Ser-314, Thr-323 and Thr-367 by phosphatase PPP2CA in an IER5-dependent manner, leading to HSF1-mediated transactivation activity (PubMed:26754925). Sumoylated with SUMO1 and SUMO2 upon heat shock in a ERK2-dependent manner (PubMed:12646186, PubMed:12665592). Sumoylated by SUMO1 on Lys-298; sumoylation occurs upon heat shock and promotes its localization to nuclear stress bodies and DNA-binding activity (PubMed:11514557). Phosphorylation on Ser-303 and Ser-307 is probably a prerequisite for sumoylation (PubMed:12646186, PubMed:12665592). Acetylated on Lys-118; this acetylation is decreased in a IER5-dependent manner (PubMed:26754925). Acetylated on Lys-118, Lys-208 and Lys-298; these acetylations occur in a EP300-dependent manner (PubMed:24581496, PubMed:27189267). Acetylated on Lys-80; this acetylation inhibits DNA-binding activity upon heat shock (PubMed:19229036). Deacetylated on Lys-80 by SIRT1; this deacetylation increases DNA-binding activity (PubMed:19229036). Ubiquitinated by SCF(BTRC) and degraded following stimulus-dependent phosphorylation at Ser-216 by PLK1 in mitosis (PubMed:18794143). Polyubiquitinated (PubMed:24581496). Undergoes proteasomal degradation upon heat shock and during the attenuation and recovery phase period of the heat shock response (PubMed:24581496). |
参考文献
克隆号文献
 New Products
New Products




![anti-HSF1 antibody [4B4]](/upload/image/20241105/8415bc38bc270b898ee2a90369a6c7fb.jpg)